 HuMed Lifesciences
HuMed Lifesciences
 HuMed Lifesciences
HuMed Lifesciences
Researching a transition from surface fixation to 3-D bone integration — without changing established workflows.
KiiS = Kim’s Ingrowth Implant System (current R&D); also used for Kim’s Injectable Implant System in our drug-delivery roadmap.
Concept intended to support research into multi-directional fixation beyond surface contact.
Standard conical connection designed for familiar clinical protocols.
Conceptual roadmap for potential drug-delivery applications in peri-implant care research.
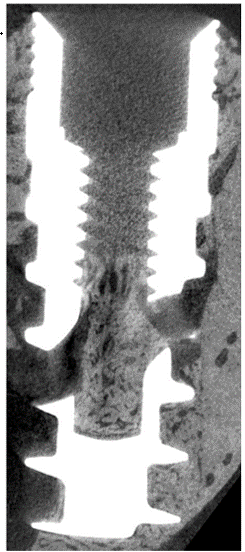
Micro-CT observations in preclinical models show bone ingrowth into the internal chamber, supporting multi-directional fixation research.
Patent listings are provided for notice only and do not imply product availability, regulatory clearance, or any license.
Regulatory Status. KiiS is a device concept under development and has not been cleared or approved by the U.S. Food and Drug Administration (FDA). It is not available for sale in the United States or other jurisdictions requiring prior authorization.
No Offer or Solicitation. Information on this website is for general informational purposes only and does not constitute an offer to sell or a solicitation of an offer to buy any securities.
Forward-Looking Statements. Certain statements may be forward-looking and are subject to risks and uncertainties; actual results may differ.